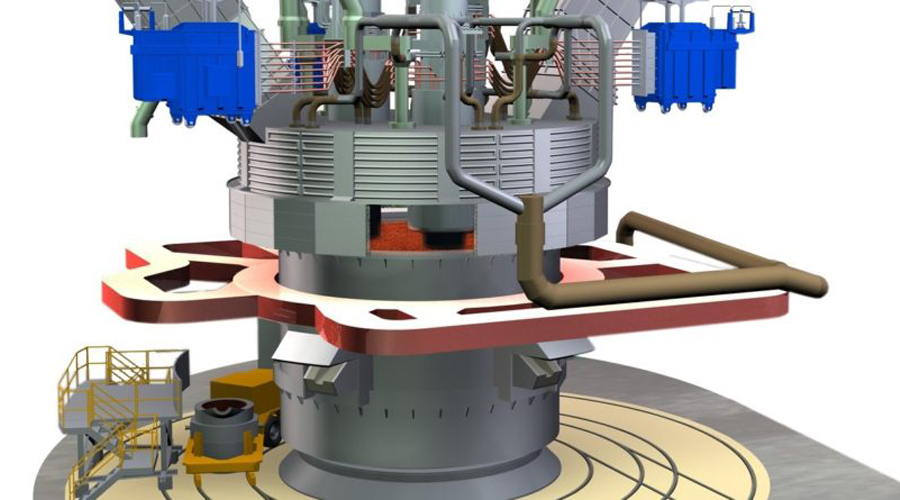
How does a submerged arc furnace work? A SAF is an electric arc furnace used in a variety of industries to produce metals such as ferroalloys, silicon, and calcium carbide. Its an important part of the manufacturing process, converting raw materials into valuable metal products.
Submerged arc furnaces operate on the principle of passing an electric current through a conductive material (called the charge) submerged in a molten bath. The bath usually consists of a combination of flux and solid reducing agent. The high temperatures reached within the furnace allow the required chemical reactions to occur to produce a specific alloy or metal.
How Does A Submerged Arc Furnace Work?
a) SAF starts with furnace charging. The charge consisting of solid raw materials is carefully added to the furnace via a charging system. The dosing system ensures controlled and precise addition of materials to maintain the desired composition of the final product. Raw materials typically include a combination of metal oxides, carbon, and other additives needed to produce a specific alloy.
b) When the charge is added, current is supplied to the furnace. An arc forms between the electrode and the charge, generating intense heat. The heat generated by the arc melts the charge and makes it part of the molten pool. The molten pool provides a highly conductive medium for current flow, ensuring efficient energy transfer.
c) During the operation of the submerged arc furnace, various chemical reactions will occur in the molten pool. These reactions are critical to achieving the desired composition and properties of the final product.
Depending on the specific alloy being produced, different fluxes and reducing agents are used to promote the desired reactions.
d) The reactions that occur in the furnace can be roughly divided into reduction reactions and slagging reactions. The reduction reaction involves the removal of oxygen from the metal oxides present in the charge, thereby forming a metal alloy. The slagging reaction involves the formation of a slag layer on top of the molten pool, which helps remove impurities and protects the metal from oxidation.
The temperature in the submerged arc furnace can reach thousands of degrees Celsius, providing the necessary conditions for the rapid occurrence of chemical reactions. The intense heat ensures thorough mixing and homogenization of the molten pool, resulting in the production of high-quality alloys.
e) Once the required reaction time is reached, the final product is removed from the furnace. The tapping process requires careful control of the flow of molten metal and slag out of the furnace. The metal is collected in ladles or molds, solidified into the required shape and form, loaded into ladles and ladle cars and pulled to the required place for use or continuous casting.
Regarding how does a submerged arc furnace work, a brief introduction is given above. Submerged arc furnaces play a vital role in the production of various metals and alloys. By harnessing electrical current and intense heat, they are able to convert raw materials into valuable products. Our understanding of the inner workings of submersible furnaces helps optimize the manufacturing process and ensure the production of high-quality metal alloys.
If you have any questions or any needs about SAF, EAF, LF, IF, CCM, etc., please feel free to contact us at HANI.